Hosting a CME Event
The Office of Continuing Medical Education (CME) is excited for the opportunity to work together planning and producing a high-quality educational event for healthcare professionals. The Office of CME staff offers a variety of services to ensure that your program is a success. We partner with you to determine your individual needs and assist in creating a plan to produce a one-of-a-kind educational experience. This coordination and teamwork is integral to the success of both the planning process and day-of event accomplishments. We offer services for the following categories of CME activities:

- Conferences accredited, planned, and managed by the Office of CME
- In-person
- Virtual
- Hybrid
- Conferences accredited by the Office of CME (event planning is not included)
- Regularly Scheduled Series (RSS)
- Enduring Materials
CME Planning Process for Events is Comprised of Five Phases:
1. Pre-Application
The Program Director and/or support staff from host department submits a pre-application to provide a high-level overview of the proposed activity. The Office of CME evaluates new activity proposals to make sure that they are consistent with the office’s mission, goals, and objectives. This preliminary evaluation also includes an assessment of whether the team is likely to have the expertise and resources necessary to successfully carry out the proposed activity. You can expect to receive a notification within 7-10 business days. Full application is required after a pre-application is approved. All full applications require approval from the applicant's department chair.
2. Consultation (for Live Conferences Only)
This phase is for live events only. Our experienced Event Planners meet with the Program Directors and relevant staff to discuss their goals and vision for the event as well as to provide a detailed overview of roles and responsibilities.
3. Full CME Application
Full application is the primary vehicle used to direct and organize the planning process so that all important program planning elements are addressed and ACCME/MSV requirements are met. The Office of CME uses a comprehensive web-based full application for CME approval. Essential components of the full application are:
- Gaps analysis (At least two evidence-based resources are required to identify gaps and needs for the proposed intervention)
- Target audience
- Learning Objectives and planned impact of the activity
- Desirable physician attributes/Core competencies
- Barriers to implementation
- Disclosures and COI (Planner disclosures are required with the pre-application. Faculty disclosures are required at least one week prior to the event.)
- Supporting documents (Agenda, list of suggested speakers, etc.)
- Projected Budget (For Conferences)
- Marketing collaterals (For Conferences)
- LOA for commercial support (if applicable)
All Full Applications require approval from the applicant's department chair.
4. CME Application Approval
Full CME applications are diligently reviewed to ensure completion and compliance with ACCME accreditation criteria as well as Standards for Integrity and Independence in Accredited Continuing Education. Once deemed complete, robust, and compliant, applications are approved by the Director of CME and Department Chair or Service Line President.
*If applicable, the rush fee for applications, an additional $500, will be charged upon approval.
5. CME Event Delivery and Evaluation
The Office of CME team works closely with program directors to determine the best delivery options. All CME activities are evaluated at least once in a calendar year.
CME Planning Fees:
The Office of CME offers an array of services and administrative fee is collected to defray the cost of logistical planning, coordination, event management, and compliance review by staff. Fee varies based on the format and scope of activity.
Download the CME Fees - ![]() 2024_Policy_13_Fees.pdf
2024_Policy_13_Fees.pdf
Format | Pricing | Description of Services Included |
Live, In-Person Event (Full-service Planning) |
| It includes processing and approval of CME application, speaker management including collection of disclosures and mitigation of relevant conflicts of interest, creation and distribution of marketing collaterals, management of finances, registration, exhibits, fundraising, evaluation, credit claiming, on-site support, and record retention for six years as required by ACCME.
|
Live, Virtual Event (Full-service Planning) |
| It includes processing and approval of CME application, speaker management including collection of disclosures and mitigation of relevant conflicts of interest, creation and distribution of marketing collaterals, management of finances, management of virtual platform (Zoom webinar), registration, exhibits, fundraising, evaluation, credit claiming, live/virtual support, and record retention for six years as required by ACCME.
|
Hybrid Add-on |
| It includes all Line In-Person services in addition to Live, Virtual services.
|
Activity Credit Fee for One-offs (CME accreditation only, event planning not included) |
| It includes processing and approval of CME application, collection of disclosures, mitigation of relevant conflicts of interest, review of marketing collaterals, collection of commercial support LOAs (if applicable), awarding credits, and record retention for six years as required by ACCME. Planning and/or event management services are not included.
|
Joint Providership Application Fee (in addition to activity credit fee) |
| It applies to nonaccredited organizations seeking to offer AMA PRA Category 1 Credit™ in collaboration with the Office of CME (the accredited provider). Additional fees will be charged based on requested services (full-service event planning or activity credit fee).
|
MOC Add-on |
|
|
Enduring Material Fee (max. 1 year) |
|
|
Rush Fee (45-30 days before the event date) |
|
|
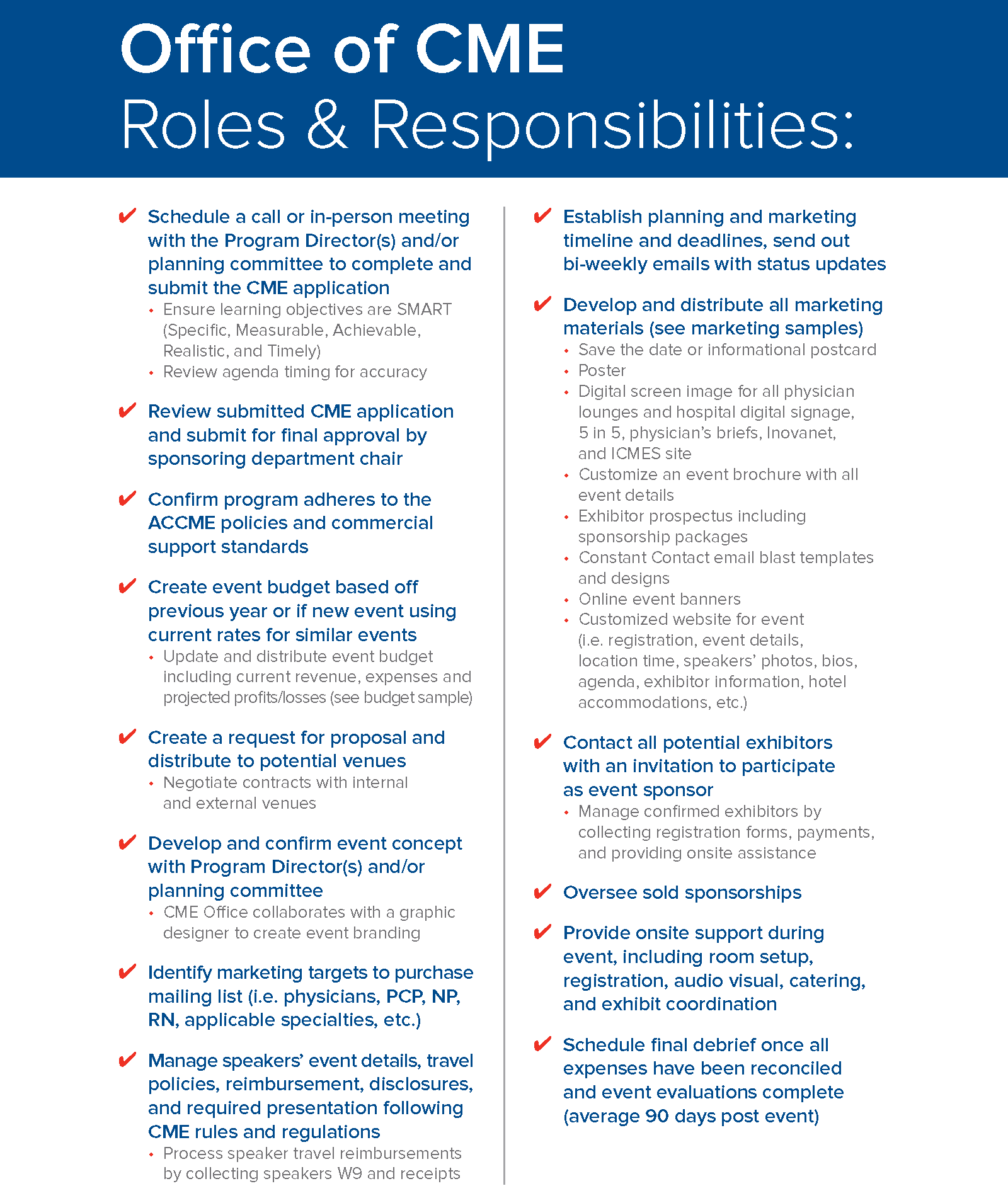
Roles and Responsibilities to Produce a Successful Live Conference:
A successful event is directly related to the partnership between the Office of CME and the Program Directors. See our Welcome Guide to see roles and responsibilities, marketing and budget examples - ![]() Inova CME Planning Guide.pdf
Inova CME Planning Guide.pdf

Questions?
Please contact us at [email protected] for more details.

 Facebook
Facebook X
X LinkedIn
LinkedIn Forward
Forward